

IMAGINE THIS ... You have been using a specific medication to treat a certain set of symptoms for several years. Now, you’ve learned that the medication has been approved for a different indication, or use, altogether. You wonder – is this medication the same as it has always been, or is it a new formulation of the same drug?
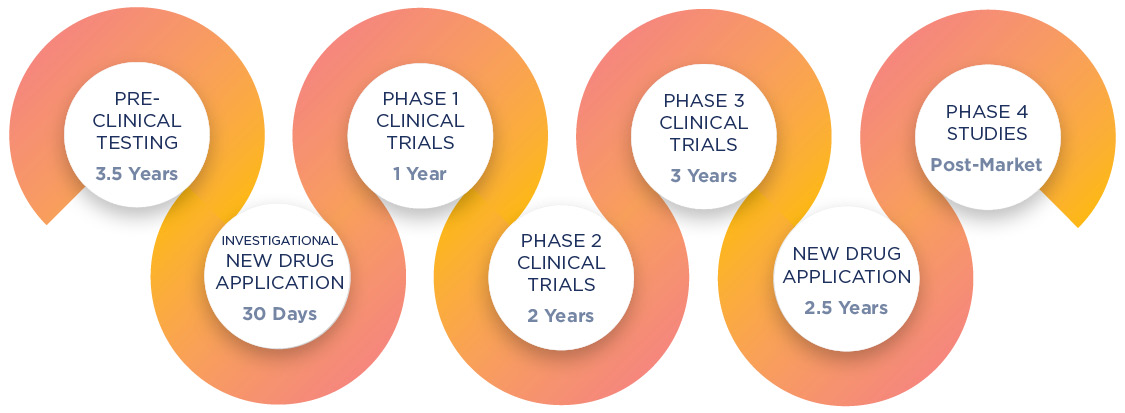
How Do New Medications Come To Market?
Prior to being available to the public, medications undergo a long approval process that is governed by the U.S. Food and Drug Administration (FDA). Rigorous research and testing are completed in order to prove that the drug is safe and effective for its intended use. On average, this process takes 12 years to complete.
Marketing Drugs
Manufacturers can only market their drug for use in treating its FDA-approved intended use, also known as its indication(s). n For example, if a drug has only been FDA approved to treat psoriasis, the manufacturer cannot market that drug to treat another condition, such as eczema.
New Indications
In some cases, medications are used to treat conditions other than originally approved. This practice, known as off-label drug use, is legal and common. Scientific evidence may suggest that a medication is both safe and efficacious in treating the prescribed condition. In addition, other beneficial uses of approved drugs may be discovered after the FDA’s initial approval of the drug. This can lead to the FDA’s approval of a new indication. Manufacturing companies are able to seek approval of a new indication for an already-approved drug. The FDA requires a supplemental new drug application (sNDA) that consists of the same quality and content as the drug’s original NDA. It is reviewed and assessed, just as the original was before it. If full approval from the FDA is granted, the drug will now carry that new indication and may be marketed to treat such condition(s).
- Returning to the example mentioned earlier – a manufacturer whose drug was previously approved for psoriasis only, can submit an sNDA for eczema to be an approved indication based on the clinical and scientific data they have.
Is There a Change to the Medication?
When a medication is approved for a new indication, there is not a change in the formulation of the drug. Rather, it is the same exact drug, but now approved for use in multiple conditions or indications.
How Can Noble Help With New Drug Indications?
Noble Health Services has a knowledgeable, dedicated team of pharmacists ready and able to help with any questions that you may have regarding a new indication on a medication that you may currently use or be considering using. In addition to being able to answer these questions, you can count on our pharmacists and entire team to:
- Provide ongoing patient monitoring for medication safety, efficacy, and adherence
- Be available 24/7/365
- Provide extensive patient education
- And more
To download and print this issue of The Noble Standard, click here.

